


Alvedon, part 2 of 2.
First, something general about pain, before we talk about that particular painkiller Alvedon. When we accidentally cut or hit ourselves, a lot of arachidonic acid arises in that area. This is a polyunsaturated fatty acid (here there are double bonds between the carbon atoms) that has a total of 20 C, and 4 double bonds. Arachidonic acid is released from the cell membranes of the affected cells and interacts with any of the enzymes COX-1 or COX-2. The former convert prostaglandins into prostacyclins. They are produced in endothelial cells (cells that are located mostly on the outside of certain organs), and the prostacyclins in question protect the gastric mucosa. COX-2 is involved in site-bound, relevant inflammations. Prostaglandins are fatty acids that cause smooth muscle to contract, and these control e.g. blood pressure, but also the body's inflammation reactions. Alvedon is a COX-2 inhibitor while inhibiting special neurotransmitters in both brain and body.
What Alvedon does is that it affects the hypothalamus (on the underside of the midbrain) so it overrides that prostaglandins create fever in the body (which is debated if it is good, there are benefits to fever. Bacteria are more sensitive than we think, and they will die if they are in a 2 degree warmer environment). Antipyretic (less fever) in this case, affects the thermoregulation of the central nervous system to the patient's advantage in the moment. The Chemical Structure of Alvedon also makes it possible to make OH^- and O^- radicals harmless. The former is easily confused with the hydroxide ion that attracts H^+, and then creates negative charges in its surroundings. Hydroxide ions, OH^(-) are always present in alkalined liquids, and this is a negatively charged compound (and therefore takes up protons) and causes some of the body's molecules/proteins to break. Hydroxyl radicals are an uncharged OH^(-): Fe^(2+) + H2O2 ---> Fe^(3+) + •OH (hydroxyl radical) + OH^(-)
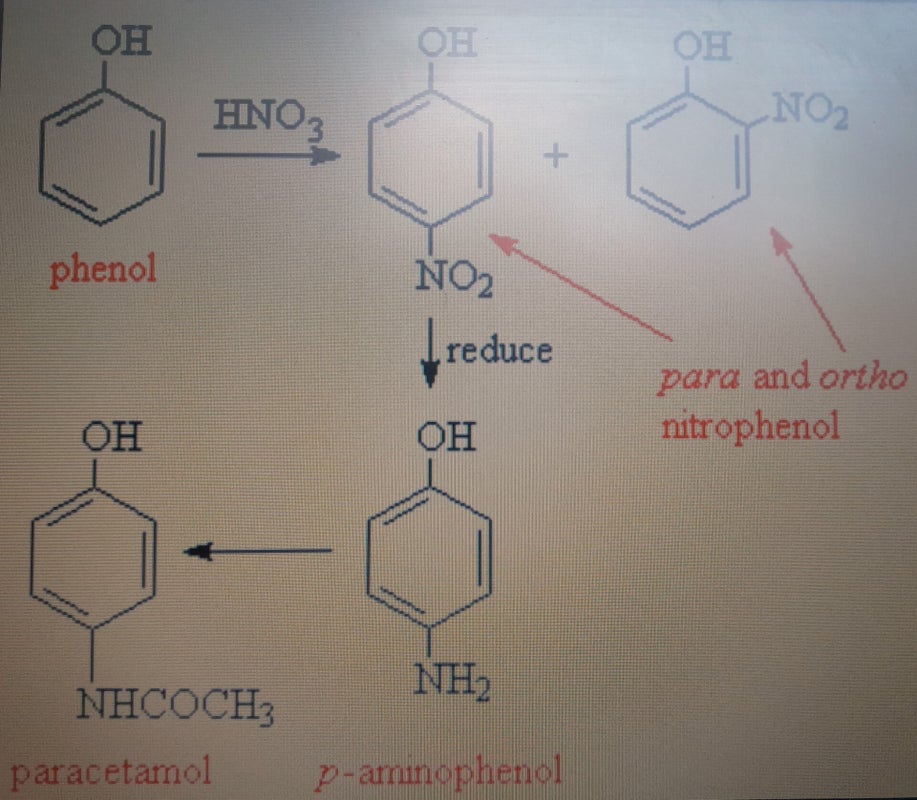
Alvedon is an analgesic (less pain), and paracetamol (acetaminophen), unfortunately, does not appear by natural means. The original painkiller was acetanilide, C8H9NO (which looks almost like acetaminophen, but it has no -OH group that attaches to the benzene ring), and Paracetamol was discovered by chance, but experiments then showed that the latter is less toxic. Acetanilide is to some extent divided into both paracetamol and aniline (which is toxic) in the body. Paracetamol is thus an acetanilid derivative (unlinking). The latter is produced like this: C6H5NH2 (aniline) + (CH3CO)2O (ethanoic anhydride) ---> C6H5NHCOCH3 (acetanilide) + CH3COOH (acetic acid). There are many different acid anhydrides, and another example is the acetic anhydride, (CH3CO)2, where the starting acid was acetic acid. Anhydrides are obtained by condensation in the lab, and two acyl groups are joined by one oxygen. These have high reactivity and are often used as reagents in syntheses.
Something else regarding the origin of acetaminophen is about the molecule phenacetin, C10H13NO2. Acetaminophen can also be made from this chemical compound, and not just from acetanilide. It is not uncommon to split phenacetin's ether in the laboratory, to get the drug in question. When it comes to pharmacokinetic interactions (which are about absorption rate), for example, liver damage can be more easily achieved if you are already taking enzyme-inducing medication. It becomes intoxication when paracetamol is taken up very quickly. You suddenly need a lower dose of paracetamol. How is acetaminophen toxic to the liver? After ingesting the drug, it will undergo a reaction in the body's liver (in the chemical factory with many different enzymes), and the -OH (hydroxy group) that attaches to the benzene ring will lose its hydrogen, H, and a double bond to the oxygen will happen, and it is the small part of the molecule that is harmful to health.
Why is the double bond to oxygen harmful to us? Can the drug technician get hurt by an inhalation? The double bond to the solitary oxygen causes the emergence of reactive oxygen species (ROS). There is an oxidative stress inside the body's mitochondria, i.e. that reactive oxygen damages the body's cells. N-acetylcysteine, C5H9NO3S, is a known antidote but it has its limitations. This must be taken at an early stage if it is to work. A few words about the manufacture. In pharmaceutical factories, e.g. a premixing and a dry mixing happens, and one wants to achieve homogeneity and therefore chemical powders are mixed several times. For example, excipients are included that fill out the tablet, in addition to the drug substance (API). With a special wet granulation (when you build up granules with liquid, but the water is removed later) the powders will dust less! It would be interesting to know if they use wet granulation in the manufacture of Alvedon in the industry.

https://www.bing.com/videos/search?q=why+we+have+pain+and+how+to+kill+it+youtube&view=detail&mid=1396F34882391A104D351396F34882391A104D35&FORM=VIRE (2023-02-20)
https://svaren.nu/vad-ar-prostaglandiner/ (2023-02-21)
https://unacademy.com/content/question-answer/chemistry/what-is-the-meaning-of-nascent-oxygen/#:~:text=Nascent%20oxygen%20refers%20to%20that%20oxygen%20atom%20which,we%20inhale%29%20and%20triatomic%20%28O3%2C%20also%20called%20Ozone%29. (2023-02-23)
https://kurera.se/fria-radikaler-och-antioxidanter/ (2023-02-23)
https://www.greelane.com/sv/science-tech-math/vetenskap/acid-anhydride-definition-606344/#:~:text=Vad%20%C3%A4r%20en%20syraanhydrid%3F%2021%20Oct%2C%202019.%20En,acylgrupper%20f%C3%B6renade%20med%20varandra%20genom%20en%20syreatom%20. (2023-02-26)
https://sv.thpanorama.com/articles/qumica/anhdridos-de-cido-cmo-se-forman-frmula-aplicaciones-y-ejemplos.html#:~:text=Allm%C3%A4n%20formel%20Den%20allm%C3%A4nna%20formeln%20f%C3%B6r%20syraanhydrider%20%C3%A4r,p%C3%A5%20liknande%20s%C3%A4tt%20f%C3%B6r%20m%C3%A5nga%20andra%20liknande%20syraanhydrider. (2023-02-26)
https://pubmed.ncbi.nlm.nih.gov/29753208/ (2023-02-27)
https://sv.wikipedia.org/wiki/Oxidativ_stress (2023-02-27)
https://en.wikipedia.org/wiki/Acetylcysteine (2023-02-27)
Add comment
Comments